Probing the dynamic structure of PmIII complexation in aqueous media by using ab initio molecular dynamics simulations
The final lanthanide was studied after establishing promethium coordination and bond lengths. The locations and intensities of the main features correspond to the Ln – O, according to the results of the Fourier transform-EXAFS. This is expected on the basis of the different harmonics generated from the shortening of the inner-sphere bonds caused by the Ln contraction. Furthermore, the shrinkage of the Ln–O bonds is corroborated by the trend in the relative energy positions of the Ln L3-edge XANES spectral features (Extended Data Fig. 3b), The results of a recent study 39 on some isostructural Ln compounds were consistent with those results.
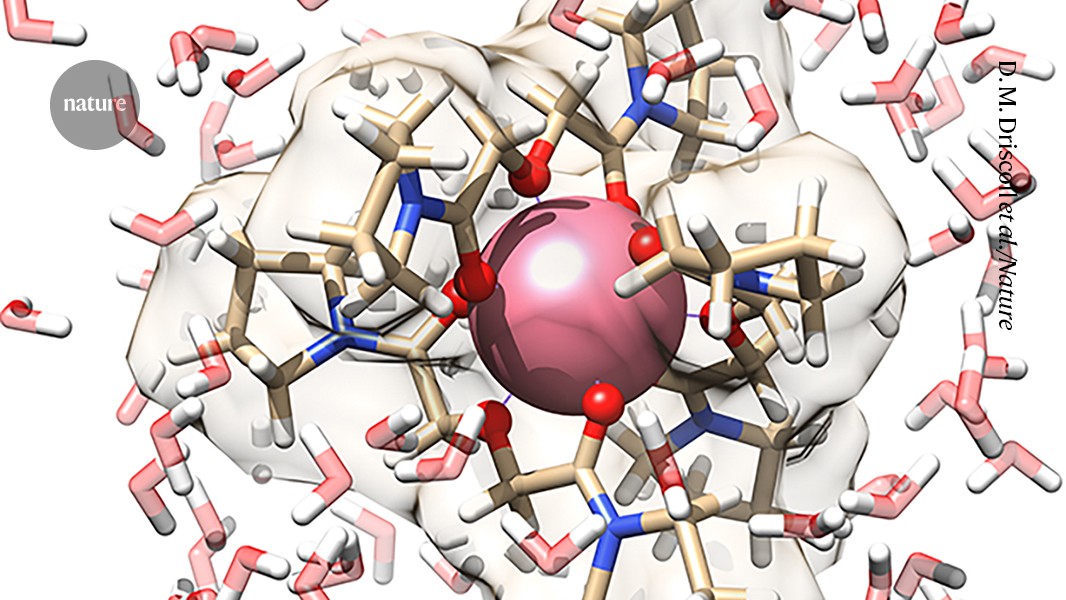
To further gain insights into the dynamic structural behaviour of PmIII complexation in an aqueous environment, we performed ab initio molecular dynamics (AIMD) simulations. The theoretical EXAFS spectrum and its Fourier transform (Fig. 2b,c) were simulated directly from the AIMD trajectory and show very good agreement with the experimental data, validating the formation of a homoleptic [Pm(PyDGA)3]3+ complex (Fig. 2d). Key structural parameters align well with those determined by the EXAFS experiments, as can be judged from the analyses of radial distribution functions (RDFs), with the AIMD predicted Pm–O bond length of 2.48 Å (Extended Data Fig. 5). Beyond the inner-sphere Pm–O correlations, the AIMD results also indicated some water structuring around the complex at 4.43 Å through transient hydrogen bond interactions with the O donor groups of the PyDGA ligands. It is also worth noting that, like in the experimental EXAFS data, the amide carbonyl and etheric Pm–O bonds could not be resolved in the AIMD and thus appeared as a single peak in the corresponding RDF, pointing to the dynamic nature of the first-sphere ligand-metal interactions in aqueous solution (Supplementary Video 1).
Spectral separation of rare-element lanthanides and actinide elements using coordination complexes containing three electron-rich atoms
“It’s a tour de force,” says Polly Arnold, a chemist at Lawrence Berkeley National Laboratory in Berkeley, California, who was not involved in the research.
Many of the rare-earth elements are valuable for their uses in technology, including lasers and powerful magnets. Although many rare earth elements are abundant in Earth’s crust, they are thinly spread and hard to find. They have remarkably similar chemistry, which makes isolating lanthanide from the rest of it difficult.
Current separation methods often use molecules known as ligands to bind to positively charged lanthanide ions in solution, forming coordination complexes. Chemists can then exploit subtle differences between these complexes to separate them: for example, by selectively washing the complexes out of water using organic solvents. Ilja Popovs, the Oak Ridge chemist who co-led the research says that you need a lot of separations to get to the pure material.
Promethium has been something of a closed book for researchers working on improved separation methods. Chemists have made a few promethium compounds, but never a complex that shows how promethium can bond to the ligands in solution.
The team combined these ions with a ligand called bispyrrolidine diglycolamide, which contains three electron-rich oxygen atoms. The three ligands hugged each promethium ion, generating complexes withoxygen bonds.
Researchers measured the average length of these bonds using X-ray absorption and theoretical simulations. They also found that oxygen forms the bonds by providing pairs of electrons that neatly fill empty energy levels, known as orbitals, around promethium.
“It’s just incredibly difficult, skilful work, and it’s really impressive that they’ve been able to do it,” says Arnold, who studies lanthanides and their heavier cousins, the actinide elements.
Each element gains one electron and a single protons at every step, from lanthanum to lutetium. Protons add to an atom’s nucleus, while electrons add to its orbitals. With lanthanides, the electrons gradually fill up a particular set of electron orbitals known as 4f that are rather diffuse and therefore do not ‘shield’ the other negatively charged electrons in an atom from the growing positive charge of its nucleus. This allows the nucleus to exert a stronger pull on some orbitals, and contract the atom more than otherwise expected.