B cells silence SHM during inertial cycling: a model for the post-mitotic phase of cellular cycle re-entry
In conclusion, our data support a model in which B cells silence SHM during inertial cycling, so as to undergo one (or possibly a few) SHM cycles per DZ passage, as opposed to one round per cell cycle. This model reconciles two salient features of GC biology—cyclic re-entry and clonal bursting—and explains how GCs can simultaneously achieve the efficient clonal expansion and sequence diversification that are required for antibodies to affinity mature.
A comparison of B cell gene expression at 48 and 72 h after anti-DEC-OVA treatment ruled out downregulation of Aicda (which we confirmed by flow cytometry using an AID-GFP fusion reporter31) as the mechanism for this pause in SHM (Extended Data Fig. 5a,b. Moreover, expression of downstream effectors of SHM (Ung, Neil1, Neil3, Apex1, Apex2, Msh2, Msh6, Polh, Hmces and Fam72a; refs. 32,33,34,35,36,37,38) was generally higher rather than lower at 48 h after anti-DEC-OVA treatment, although fold changes were modest (Extended Data Fig. 5a). There was also no detectable downregulation of Igh mRNA or of Jh4 intronic RNA (a real-time indicator of Ighv transcription), suggesting that transcription of the Ighv region, a requirement for AID targeting39, is intact during inertial cycling (Extended Data Fig. 5c). A sharp decrease in the proportion of B cells assigned to the G0–G1 phase of the cell cycle could be seen by the correlation between inertial cycling and this. 5d). This is in line with our previously published findings in the Ccnd3T283A mutant21 and indicates that B cells spend less time in the early phases of the cell cycle while undergoing inertial divisions. Given the established restriction of SHM to the earlier cell-cycle stages40,41,42, we hypothesized that B cell lineages undergoing clonal bursting in the DZ would be unable to undergo SHM except during the final post-mitotic phase that takes place between the end of inertial cell cycling and return to the LZ. It would be equivalent to undergoing one round of SHM per DZ passage, rather than one per cell division. The duration of time B cells spent in G0–G1 under normal or clonal-burst type cycling was sought to measure the hypothesis.
The CDK2 activity is low in the G1 and G0 phases of the cell cycle, which promotes progression through S-phase initiation. To measure these phases precisely in vivo, we generated mice carrying a real-time reporter of CDK2 activity (Fig. 3b,c). A reporter was written based on constructs 43, 44, which consists of the fused DHB and the tdTomato red fluorescent protein. The nucleus is translocate from the DHB domain after CDK2 is activated. It is seen in the nucleus when CDK2 activity is absent but not present in G0 and gradually exiting to the cytoplasm during G1, S and G2 phases. 3a,d This localization-based readout for CDK2 activity, determined as the cytoplasmic-to-nuclear (C/N) ratio of DHB–tdTomato fluorescence (Fig. 3b), allows for monitoring of cell cycle progression with greater time resolution than can be achieved using conventional genetically encoded cell-cycle indicators46 that require transcription, translation and maturation of fluorescent proteins to take place before a signal can be detected. A histone 2b (H2B)-eGFP reporter47 was crossed to the mouse to make it easier to visualize the events that occur.
To experimentally test whether mutation rates are variable, and dependent on both affinity and division rates, we tracked GC B cell division in mice that express mCherry labelled Histone-2b (H2b-mCherry) under the control of a doxycycline (DOX)-sensitive promoter23,28. Lymphocytes from these mice (H2b-mCherry mice) constitutively express the mCherry indicator. Administration of DOX turns off the reporter gene and, upon dividing, cells dilute the indicator in proportion to the number of divisions made, whereas quiescent cells retain the indicator22,23,28 (Extended Data Fig. 1b–c.
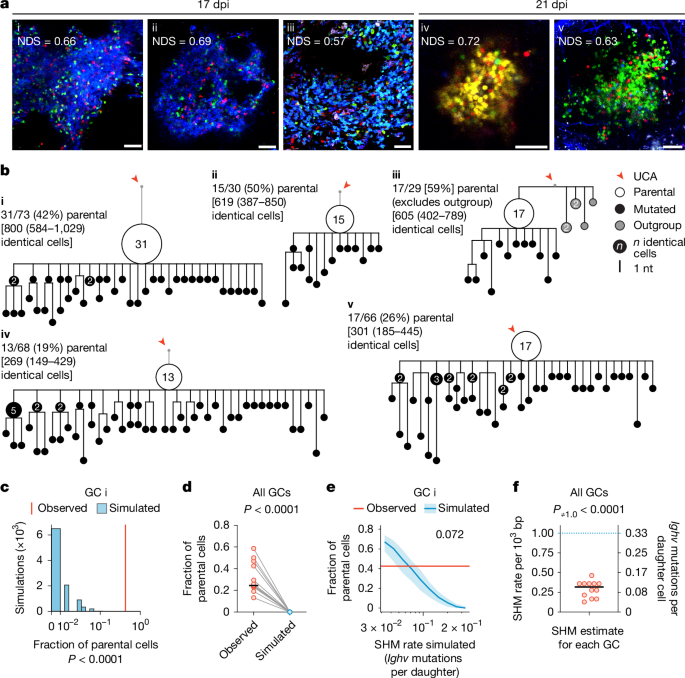
Our modelling predicts that a constant mutation rate of around 1 × 10−3 per base pair per cell division1, equivalent to approximately 50% chance of mutation per division pmut = 0.5, should produce branched trees containing limited small collections of identical sequences (nodes) of 15 or fewer cells (Fig. Black, 1c. Alternatively, the decreasing pmut model, with otherwise identical simulation parameters, predicted trees with nodes that extended up to 50 cells (Fig. 1c, red. The trees with the highest number of members were also the trees with the highest number of identical members. 2f). These grossly expanded nodes (more than 15) could not be accounted for by the constant pmut model but were more consistent with the decreasing pmut model.
Immune responses to simple haptens like NP might differ from more complex protein antigens. To determine the contribution of mutation-free clonal bursts to immunization with a vaccine antigen, we performed single-cell analysis of GC B cells obtained from draining lymph nodes of H2b-mCherry mice immunized with the receptor binding domain (RBD) of SARS-CoV-2 in adjuvant (Extended Data Fig. 3a). GC B cells obtained 14 days after vaccination, and 36 h after DOX exposure were barcoded according to RBD-binding and mCherry expression, allowing paired analysis of sequence identity, division status and RBD-binding as a surrogate for affinity (Extended Data Fig. 3b. The trees were created with the use of IgH and IgL-chain sequence from expanded clones. The annotations included the cells’ mCherry expression and the pink and black outlines of the RBD-binders. Similar to NP-ova immunization, we observed large nodes containing identical sequences which showed that the vaccine caused extensive bursts of genetic modification. The number of nodes that had 1, 2–15 and more than 15 identical sequences was used for the fraction of clones that it represented. When all cells are considered independently, a large number of GC B cells are derived from cells that carry more than 15 identical words. 3d and Supplementary Table 5). The fraction of nodes containing more than 15 identical sequences was always greater amongRBD+ compared toRBD cells.
Finally, we considered whether stochasticity in the constant pmut model could account for the large nodes observed in experimental data. To this end, we simulated the case with stochastic pmut (Extended Data Fig. The case when the number of divisions related to a constant level of T cell help was known as stochastic. 7b). We found that neither scenario could account for the long-tail behaviour of node sizes observed in experiments. Thus, both the theory and experimental data are consistent with the idea that the per division rate of SHM is regulated and decreases with increasing T cell help.
The C>U mutations are introduced at preferential nucleotide sequence hotspots (WRC, W, A/T, R, A/G). To determine whether absence of SHM in expanded nodes was due to previous loss of these motifs, we profiled AID hotspots between cells contributing to nodes of sizes 1, 2–15 or more than 15. All cells belonging to the three classes of nodes had the same motifs as the other ones. 8d–f Differences in SHM between the two components are not accounted for by target motif decay. Finally, we compared the level of cell death among mCherry compartments and found no significant differences (Extended Data Fig. 8g,h
Together these data are consistent with the idea that strong selection signals decrease the relative time DZ cells spend in G0/G1, thereby reducing their exposure to AID and lowering their per division mutation rates.