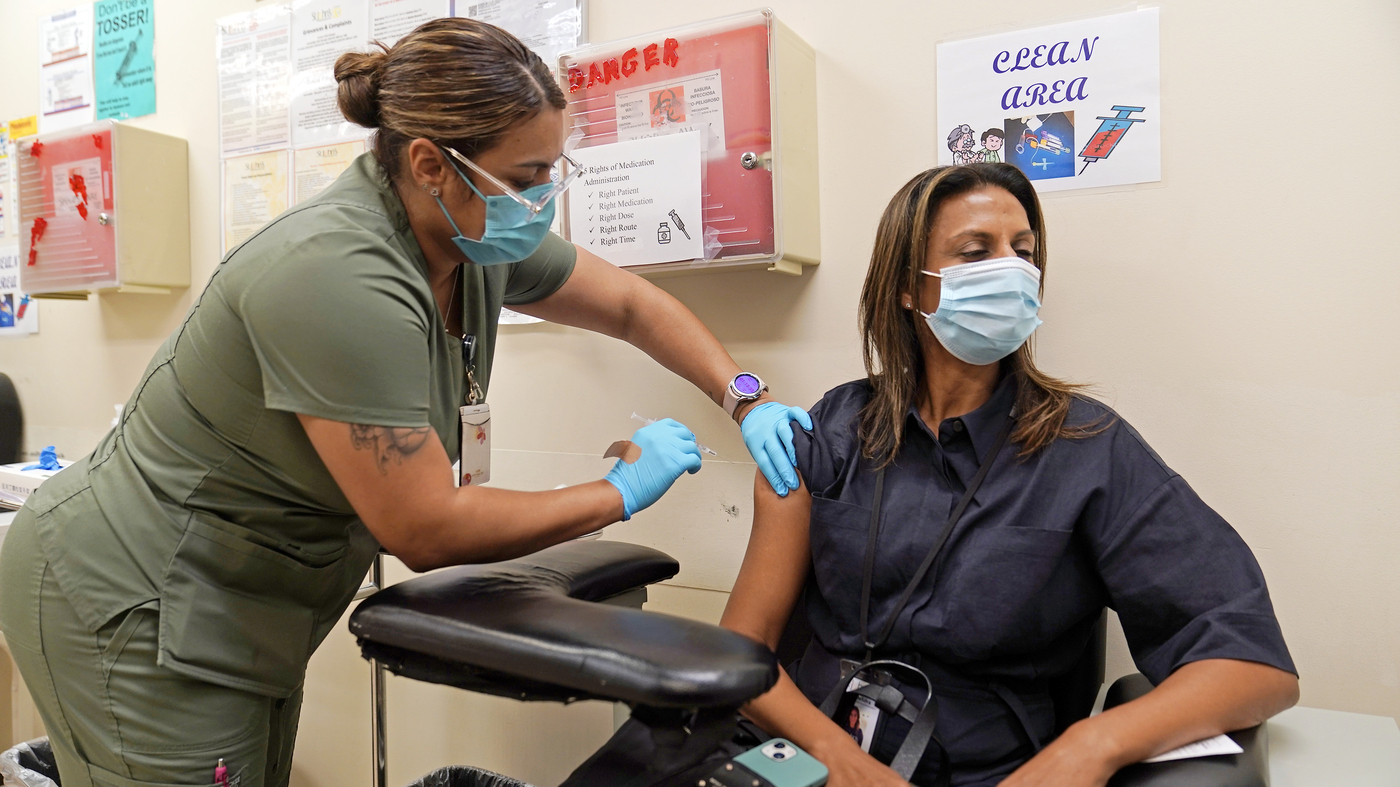
The Effects of Booster Vaccination on Covid-19-Associated Hospitalization in Older Adults and the Seasonality of SARS-CoV-2 Spread
According to a study by The Commonwealth Fund, if booster vaccinations continue, there could be as many as 1,029 Covid-19 deaths per day this winter. On average, more than 400 daily Covid-19 deaths occur in the United States.
The message is simple: Don’t wait. Get vaccinated. Go get vaccinated now; get it before Halloween so you are ready before Thanksgiving and Chrismas and the holidays,” Jha said.
The study shows that a bivalent booster dose provided strong protection against COVID-19-associated hospitalization in older adults and additional protection among people with previous monovalent-only mRNA vaccine. “All eligible persons should get a bivalent booster to maximize protection against COVID-19 hospitalization this winter season.”
The timeline for updating flu jabs is based on the well-documented seasonal pattern for the emergence of new strains: selection of strains for Northern Hemisphere vaccines is based in part on which versions spread widely during the previous Southern Hemisphere winter. The seasonality of the spread of SARS-CoV-2 is not as predictable as the seasonal spread of influenza.
But the CDC came out with two studies Friday detailing the bivalent vaccine’s effectiveness against COVID-related emergency department visits and hospitalizations and effectiveness against hospitalization specifically among older people.
The study looked at 27 million older Americans and others that are in a Medicare fee-for-service health plan. It included data from the Delta and Omicron surges last year.
Implications of the Omicron B.4/BA.5 Subvariant on the Immune Responses of Children and Adults Vaccined with Covid-19
With the report we hope that Americans will be updated with their vaccinations so that they are prepared for the fall and winter.
Becerra said the administration continues to urge people to get the updated Covid-19 boosters now available for ages 12 and up. The updated boosters for children as young as 5 years old have been requested by Moderna and Pfizer.
Pfizer and BioNTech said Friday that the immune responses against Omicron BA.4/BA.5 subvariants were “substantially higher” in people who got its new bivalent booster compared with people who received the companies’ original Covid-19 vaccine.
The Omicron BA.5 subvariant had dominated US Covid-19 infections since July, but a mix of other Omicron subvariants have been gaining against it. BA.5 According to the US Center for Disease control and Prevention, now accounts for 49.6% of new infections in this country.
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Pfizer CEO Albert Bourla said in a statement. The updated data gives confidence in our ability to rapidly update the vaccine to match the most prevalent strains every season, as well as our ability to adapt the platform.
Pfizer and BioNTech are still conducting larger clinical trials of the updated boosters, and said they are continuing to test the vaccine against the other variants of the virus.
Older adults who got the updated booster a week or so before they were sick were less likely to be hospitalized compared to unvaccinated people. The study also wasn’t able to analyze the effect of previous infection with SARS-CoV-2.
The first study was done in seven health systems when the omicron BA. 5 variant was the most dominant variant.
Vaccination Hesitancy in Public Health: The Case for a Simplified Schedule and a Single Vaccine Composition
“I think it will be an uphill battle,” Kates told NPR in September, when she was senior vice president and director of global health and HIV Policy at the Kaiser Family Foundation. “It’s difficult to sell when we’re at this point in the Pandemic.”
In the United States, 15% of eligible people have received a two-strain jab. Some experts say that is because many people have received a mixture of vaccines, which has led to confusion over which type of booster they should get and when.
“That is very concerning,” says Claire Hannan, who helps immunization officials from all 50 states run vaccination programs as the executive director of the Association of Immunization Managers.
Part of the reason people are no longer jumping at the chance to get vaccinated is that they don’t think COVID-19 is a big risk anymore, says Cynthia Baur, who directs the Horowitz Center for Health Literacy at the University of Maryland.
“We desperately need to simplify the vaccination schedule,” says Megan Ranney, a physician and public-health specialist at Brown University in Providence, Rhode Island. “If we’re going to sustain our ability to vaccinate the country, we have to move toward a more standardized schedule, from a behavioural-science point of view.” The proposal to use a single vaccine composition for the entire series would help alleviate some confusion and it might increase vaccine take up due to the fact that jabs could be offered alongside the annual flu vaccine. “These changes make a lot of sense.”
Baur has worked with community health workers who are out in Maryland pounding the pavement, talking to people about vaccination, and it’s slow going. “I don’t think that we or anybody else doing this work has found any particular message or fact or phrase that is kind of really changing hearts and minds,” Baur says.
She says that providers are still the top source of vaccine recommendations. “If providers are recommending vaccines, at least it’s opening the door to a conversation and the likelihood that somebody might think a little bit more carefully about it.”
There are a lot of ways to combat vaccine hesitancy, including focusing on misinformation or politicization or trust in public health. Moore of Immunize.org said that he decided to look at how to improve the vaccine experience.
She pointed out that about a quarter of people are afraid of needles. How many people who are not willing to be injected are saying that they don’t want it, they don’t have time, or they don’t think it works? For how many of them is that really just an excuse?”
She says the Autism Society for America has been pioneering strategies to help families and kids with autism get vaccinated, since it can be especially stressful and upsetting for people with autism. They have some simple, low-cost ideas like putting on headphones, listening to your favorite music, or using a little plastic “shot blocker” to make the shot hurt less.
I recently tried a variation of this when I took my 7-year-old daughter, Noa, to get her bivalent booster. The fear of needles among kids is more than twice as high as adults. I bought an over-the-counter lidocaine patch (marketed for back pain) at the drugstore and cut it to fit her bicep. I stuck it on her arm before we left. I drew an outline on her skin so the immunizer could give her the shot. Noa was thrilled that the shot didn’t hurt, and she was proud she hadn’t cried. She asked if we could use it in every shot going forward.
Peter Marks, head of the FDA’s Center for Biologics Evaluation and Research in Silver Spring, said that administering a jab before winter surge could avert a rush of hospitalizations. In winter, clinics are swamped with people infected with influenza and respiratory syncytial virus (RSV), which led to some US hospitals nearing capacity this season.
Additionally, SARS-CoV-2 variants don’t sweep the world as uniformly as influenza strains do, which means it will be difficult to coordinate the composition of a COVID-19 jab globally. Bruce Gellin, a global-health specialist at the Rockefeller Foundation’s Pandemic Prevention Initiative in New York City, asked at the meeting if the annual-update proposal would implicitly require that other countries follow the FDA’s decisions. The vice president for global supply chain at Pfizer, located in New York City, was not sure if that was the case.
Some meeting panellists were concerned about the decision to use a bivalent one, rather than an updated single-strain one. Scientists noted that there are few data on the effectiveness of bivalent vaccines when given as a primary series — particularly in young children, who make up a large proportion of the people now receiving a primary series in the United States. Studies suggest that the vaccine might not be as effective against Omicron if the ancestral strain is included.
The biggest jump was in antibodies that target the original strain of the coronavirus (although that version is no longer circulating, so it’s unclear how helpful those antibodies are). Antibodies that target BA.5, which was the dominant strain last summer and fall, also increased substantially. The smallest boost was seen for antibodies that defend against some of the newer Omicron subvariants that have more antibody-evading mutations, such as BQ.1.1 and XBB (the current dominant strain, XBB.1.5, wasn’t circulating when the experiments were conducted).
“There’s a clear step down” in protection as the variants continue to progress, said Dr. Dan Barouch, a professor of medicine at Harvard Medical School who led one of the studies.
The boosters are safe in most of the cases. There is preliminary evidence the bivalent booster could raise the risk of stroke in older adults, according to a joint statement by F.D.A. and C.D.C. The recent boosted group had less strokes than normal, but that was not the reason for the lower strokes in the comparison group.
For people who are under 50 and don’t have an increased risk of severe disease, there’s more of a debate about whether another shot is worth it. The booster is still effective, but getting it is less critical.