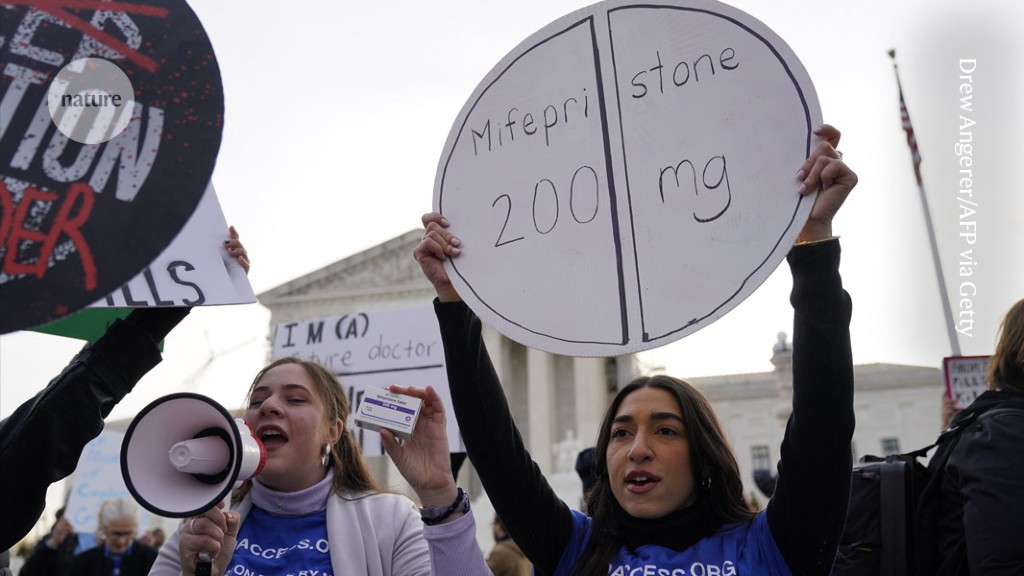The Daughter of Dobbs: The Legality of Abortion in the U.S. Court of Appeals for the Fifth Circuit
Abortion is being discussed at the Supreme Court. anti-abortion doctors are challenging the FDA’s actions that make abortion pills more accessible.
Drug can be used through 10 weeks of pregnancy, rather than the previous limit of 7 weeks, because of changes made by the agency. The changes also relaxed the requirement that the drug be dispensed in person, allowing it to be sent through the mail. If the court invalidates those actions, mifepristone access would be restricted nationwide. The Supreme Court legalized abortion nationally in 2022, after reversing the previous decision.
A large number of American women choose to end their pregnancies using a two drug combination of pills. The case is called the daughter of Dobbs because of the Supreme Court’s decision to leave the legality of abortion to the states.
Marsha Henderson, a former associate commissioner for women’s health at the FDA, said it would be traumatizing to the system.
“We have a very clear scientific approach…it’s not just a helter-skelter set of ad hoc opinions,” Henderson says. The teams of scientists and researchers that participate over many years, starting from phase one pre-clinical all the way to post market, help to enhance the world of research.
She insists that the data doesn’t support the agency’s decision to increase approval of the drug during the first 10 weeks of pregnancy, rather than the original approval of seven weeks.
Siding with the FDA in this case are virtually all of the major medical associations in the country, as well as pharmaceutical and bio-tech companies, big and small. The Alliance for Hippocratic Medicine argues that the FDA’s relaxation of restrictions is unwarranted and unsafe.
Such a decision “would have nothing to do with science or medicine or protecting the health of pregnant people,” says Heidi Moseson, an epidemiologist at Ibis Reproductive Health, a global research organization that supports abortion rights, who is based in Oakland, California.
Later that year, the US Court of Appeals for the Fifth Circuit ruled that mifepristone should remain an approved drug but invalidated the FDA’s recent actions to make it more accessible. The Fifth Circuit decision didn’t take effect immediately because the Supreme Court stayed the Kacsmaryk ruling.
In 2016, the FDA approved the use of mifepristone for up to 10 weeks of pregnancy, instead of the previous seven weeks; it reduced the required number of in-person visits to doctors from three to one, and lastly, it permitted the drug to be prescribed and dispensed not just by doctors but, in states that allowed it, also by specially certified midwives and nurse practitioners.
In April 2021, at the height of the pandemic, the FDA temporarily dropped the in-person dispensing requirement, citing the health emergency. That allowed patients, who previously had to go to a clinician’s office for their pills, to instead get their prescriptions filled at pharmacies or by mail.
Eight months later, the agency looked at data collected during the “natural experiment” created by the pandemic. It found no difference in serious adverse events whether the drug was dispensed in person or not.
The FDA relied on insufficient data to justify the changes that made it easier to purchase a medication used in abortion procedures.
The case of a baby in the hospital: A pro-life doctor’s opinion of the government, the industry, and the national assembly about food safety
The risk of a baby’s health problems going up ten fold from seven weeks to 10 weeks is because the child is larger.
There were between two and three percent of women who needed follow up care. “So they’re just wrong when they suggest that there is some kind of additional complications that were brought on by moving to 70 days.”
Hawley is adamant that they do have standing. “The fact that our pro-life doctors have oriented their practices and even their lives to avoid elective abortion procedures, I think goes to show that those doctors do have standing here.”
No matter which side prevails, a single aspect of this case is truly remarkable. One would be hard pressed to find another case in which the government regulator, the regulated industry, and even the independent watchdog group that frequently criticizes the agency are all on the same side.
The case seems to be a prime example of turning a small lawsuit into a national assembly about food safety, said justice Neil Gorsuch.
The Case for a Cumbersome New Standard for Drug Development: A Comment on “The Case of Mifepristone” by K. Kacsmaryk
Kacsmaryk relied on problematic studies to question Mifepristone’s safety. Problems with the study design and methodology were some of the issues he cited as reasons for the papers being withdrawn. The papers that Kacsmaryk cited were not in the prevailing scientific literature.
The decision could affect drug development in other ways. The Fifth Circuit called for a cumbersome and unprecedented new standard for drug approval. If the Supreme Court follows that lead, “that would create delays, that would create inefficiencies and costs and would ultimately undermine patient access to scientifically appropriate medicines,” Temkin says.