-
Rethinking views on Alzheimer’s disease

Scientists have developed a drug that could stop the progression of Alzheimer’s in a patient with a mutated copy of the APOE3 gene. Piedrahita Piedrahita had two copies of the most common version, APOE3. Her variant was called APOE3 Christchurch, or APOE3Ch. This mutation affects how the protein binds to a sugar-protein compound called HSPG,…
-
Perspective on Alzheimer’s disease
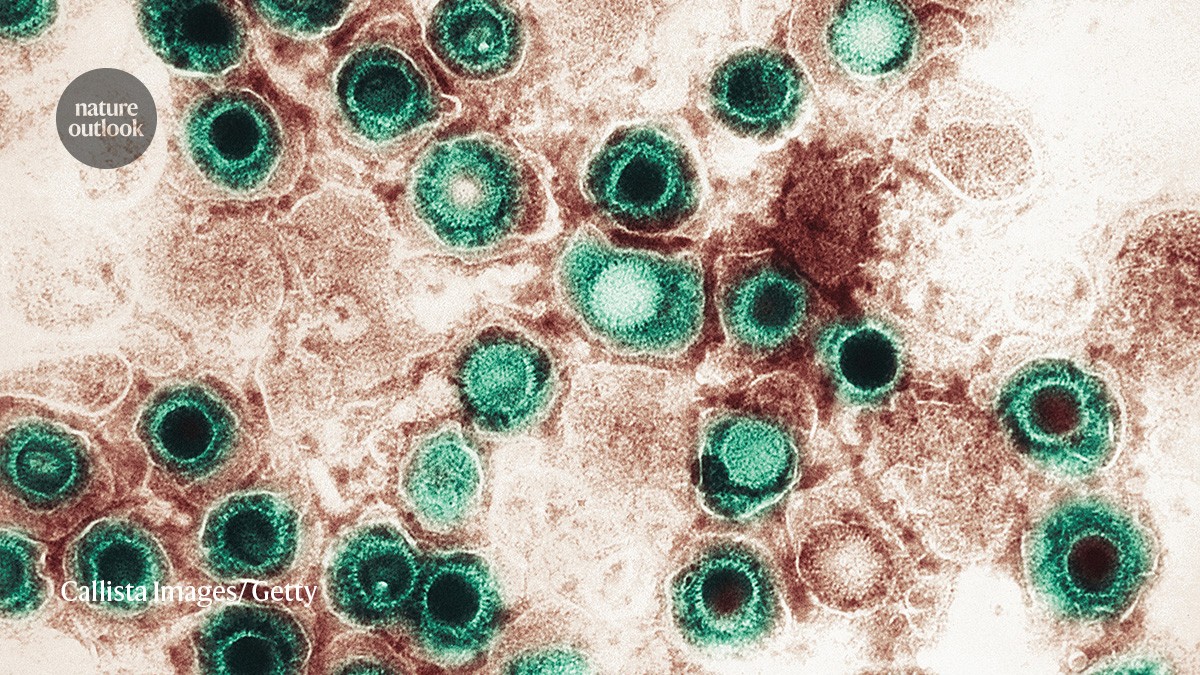
Researchers have found that familial Alzheimer’s patients had signatures of viral processes and infection in the olfactory bulb. “We showed that familial Alzheimer’s patients actually had signatures…in the bundles of nerve fibres that run to these smell centres in the brain, compared with healthy individuals,” researchers said. In 2010, they had demonstrated that amyloid- exerts…
-
New insights into their evolution are given by the long-awaited ape genomes

Researchers have developed a new technique that can help them create 3D maps of the genome of several animal species like humans. The method uses electron-powered plasma accelerators to create virtual copies of the genome. It also helps researchers gain a better idea of the genetic factors behind different diseases including Alzheimer’s and cancer, among…
-
Climate change is impacting global timekeeping

Researchers at the Albert Einstein College of Medicine found neurons use their own DNA as a signalling system to recall how climate change and the melting of the polar ice caps have affected their training. It could lead to a build-ups of errors in neuron’s DNA, potentially causing neurodegenerative diseases like Alzheimer’s, the researchers said.
-
The FDA approved a drug that slowed the growth of Alzheimer’s.

US’ Food and Drug Administration is expected to decide this week whether to give accelerated approval to lecanemab, an experimental Alzheimer’s drug, according to Biogen and Eisai. Lecanemab has been tested in a phase III clinical trial to see if it would stop brain shrinkage in Alzheimer’s patients. Earlier, three people had died after receiving…
-
Is lecanemab the key to changing the course of Alzheimer’s disease?

US’ Food and Drug Administration (FDA) is considering an accelerated approval for a drug to treat Alzheimer’s disease. Leqembi, developed by Biogen and Eisai, reduced marker of amyloid in Alzheimer’s patients’ brains and was associated with less decline in cognitive function than a placebo. The drug is currently being tested in a Phase 3 clinical…
-
The new report shared details about death that may be linked to the Alzheimer’s drug.

The Alzheimer’s Association has said it hopes the Centers for Medicare and Medicaid Services “will move quickly” to cover lecanemab for Alzheimer’s patients. “It will take clinicians some time to be available to parse out how this drug may or may not be effective in their own individual patients,” an expert said. The Alzheimer’s Association…
-
The study puts a leading theory to the ultimate test.

Alzheimer’s Association has asked the Centers for Medicare & Medicaid Services (CMS) to provide full and unrestricted coverage of FDA-approved Alzheimer’s treatments. This comes after lecanemab, a drug that removes amyloid from brain, was rejected as a treatment for Alzheimer’s. The Alzheimer’s Association said there are more than 300 Alzheimer’s treatments in clinical trials.