The C-F Functionalization of Non-Activated Trifluoroethylarenes. Part II. Observation of Degradation in Water at 600 mpa
Direct observation of the degradation of polyvinylchloride in water at temperatures and pressures greater than 700 mpa was done. J. Appl. Polym. There was a report in the science of 106, 1075–1086.
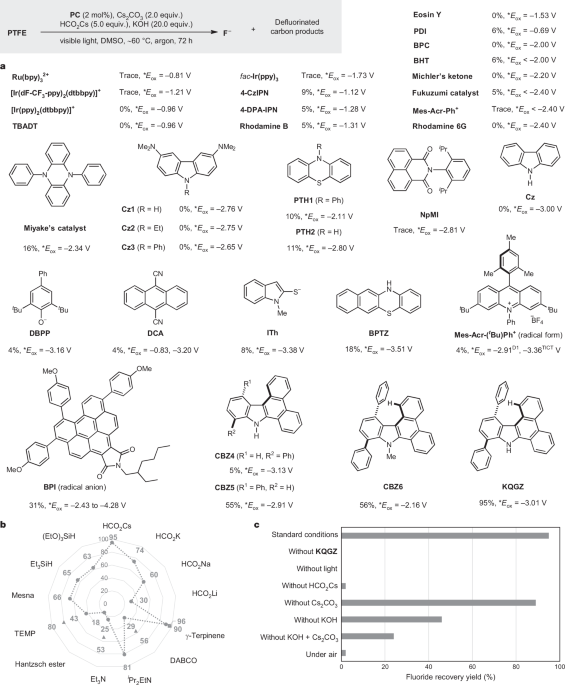
A group of people have summarized the results of the C–F functionalization of non-activated trifluoroethylarenes. J. Am. Chem. Soc. 141, 13203–13211 (2019).
The formation of a gas-phase C-F bond is achieved using W, Si, P-, and I. J. Am. Mass. The sample was analysed by a spectrometry 9, 1158–1167 (1998).
Photocatalytic reductive C–O bond cleavage of alkyl aryl ethers can be accomplished using catalysts with cesium carbonate. J. Organizational. Chem. 86, 2545–2555 (2021).
Wang, S. D., Yang, B., Zhang, H., Qu, J. P. & Kang, Y. B. Reductive cleavage of C–X or N–S bonds catalyzed by super organoreductant CBZ6. It’s an organization. Lett. 25, 816–820 (2023).
Xiao, Z. F. et al. Iniciative access to pyrrolidines and pyrroles is possible when there is aridium-catalyzed cyclization of isoxazolines. A group of people who work together. Lett. 18, 5672–5675 (2016).
Effect of Visible-Light Photoredox Conditions on the Properties of Poly(tetrafluoroethylene) and Their Reduction Potentials
The behavior of a phenothiazine is reduced in the presence of visible-light photoredox condition. J. Am. Chem. It was Soc. 35, 22403–22412-2023.
A monograph by Liang, K. Intermolecular oxyarylation of olefins with aryl halides and TEMPOH catalyzed by the phenolate anion under visible light. The chemical is Chem. Sci. 11, 6996–7002 (2020).
Kim, H., Kim, H., Lambert, T. H. & Lin, S. Reductive electrophotocatalysis: merging electricity and light to achieve extreme reduction potential. There is J. Am. Chem. Soc. 142, 2087–2092 (2020).
The photophysical properties and potentials of photosensitizers are discussed. Synlett 33, 1154–1179 (2022).
Sheldon, D. J., Parr, J. M. & Crimmin, M. R. Room temperature defluorination of poly(tetrafluoroethylene) by a magnesium reagent. J. Am. Chem. Soc. 145, 10486–10490 (2023).
Costello, C. A. & McCarthy, T. J. Surface-selective introduction of specific functionalities onto poly(tetrafluoroethylene). Macromolecules 20, 2819–2828 (1987).
The results of thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment were presented. Nature 423, 327-243 (2001).
Améduri, B. & Hori, H. Recycling and the end of life assessment of fluoropolymers: recent developments, challenges and future trends. Chem. Soc. Rev. 52, 4208–4247 (2023).
Puts, G. J., Crouse, P., and Ameduri, B. M. Polytetrafluoroethylene: synthesis and characterization. Chem. Rev. 119, 1763–1805 (2019).
Under mild catalytic conditions the complete hydrodehalogenation of polyfluorinated and other polyhalogenated benzenes could be achieved. Environ. Sci. Technol. 47, 6545–6553 (2013).
Singh, R. K. et al. Poly- and perfluorinated compounds are removed Rapidly from waste in a pilot-scale reactor. Environ. The article is titled, “Sci. Technol. 53.”
Hao, S. et al. Per- and polyfluoroalkyl substances are destroyed through a hydrothermal alkaline treatment. This is the Environ. S.h. Technol. In the year 2011, 55, 3283–3 295.
Yang, N. et al. The destruction of the chemicals in the debris by a ball milling technique. It’s Environ. Sci. Technol. Lett. 10, 198–203 (2023).
J. Gao, et al. Near-complete defluorination of chlorinated polyfluoroalkyl substances and photochemical degradation pathways. Nat. Water 1, 381–390 (2023).
Liu, Z. et al. Accelerated degradation of perfluorosulfonates and perfluorocarboxylates by UV/sulfite + iodide: reaction mechanisms and system efficiencies. Environ. Sci. Technol. A total of 56, 3699 and3709 were included.
Source: Photocatalytic low-temperature defluorination of PFASs
The Role of PFCs in the Biopolymerization of Hydrogen and Perfluoroalkyl Substituents
Gaballah, S. et al. There is an evaluation of toxicity in the zebrafish exposed to GenX and other PFAS. Environ. Health Perspect. 128, 047005 (2020).
Sunderland, E. M. et al. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. It was called the J. expo. There is a science to the topic of the Environ. Epidemiol. 29, 131–147 (2019).
Washington, J. W. et al. There is no targeted mass-spectral detection of the chloroperfluoropolycarboxylates in New Jersey soils. Science 368, 1103–1107 (2020).
Medicinal chemists are trying to make drug molecules that contain C–F bonds but can biodegrade safely once they leave the body. In some instances, PFCs have replaced harmful chemicals like chlorofluorocarbons. Non-fluorinated refrigerants are also available, including ammonia and carbon dioxide, but large-scale roll out will need regulatory input.
Such bonds lie at the heart of per- and polyfluoroalkyl substances (PFAS), a group of compounds, numbering in the millions, that are remarkably water-, heat- and greaseproof. Teflon was invented in the 1930s to make pans non-stick and keep rain out of our jackets. Varieties of cosmetics, fire-retardant foam, kitchen utensils, metal coatings, packaging, textiles and more all contain them.
Chemical catalysts based on light absorbers and a new method to clean up waste water and soils in the United States and other countries
The world has started to act to stop chemicals from entering the environment and clean up those already there. There is need for more action.
Both methods combine a catalyst with some relatively simple chemistry driven by the energy of visible light. In each case, the catalyst absorbs light that then triggers a reaction.
Chemist Garret Miyake at Colorado State University in Fort Collins and his colleagues use this absorbed energy to reduce the C–F bond to carbon–hydrogen — albeit not in Teflon1. The energy from this is used to break the bond and the molecule down to smaller parts at temperatures as low as 40 C. Both papers mark a huge step forward.
Next steps include using these ideas to develop catalysts that work in waste water or that can be used to clean up contaminated soils. If a method can be adapted so it is powered by sunlight, that would be of huge benefit.
The latest list of banned substances for persistent organic pollutants was reviewed by the international agreement that banned them.
The European proposal doesn’t yet extend to banning PFAS in applications such as medicine or transport, for the simple reason that these chemicals are still too useful and adequate alternatives are yet to be found. C–F bonds in pharmaceuticals allow molecules to remain stable, which is necessary for products’ shelf life.